Abstract
Introduction Acute myeloid leukemia (AML) is a clonally heterogenous group of disorders with many treatment options. Despite the increase in available options including targeted therapy, challenges remain in identifying patients who may respond to a given regimen. The ability to accurately predict responses to treatment is crucial given the varying response rates to standard treatments, adverse effects and increasing drug cost. In this study, we explore the ability of an ex vivo combination drug sensitivity platform, quadratic phenotypic optimization platform (QPOP), in identifying potential responders and non-responders to possible combination therapies in FMS-like tyrosine kinase-3 (FLT3) mutant AML. We further investigate the mechanism behind selective sensitivity and resistance to FLT3 inhibitor-based combinations.
Methods FLT3-ITD mutant or WT AML cell lines were treated with a set panel of drugs recommended by physicians including FLT3 inhibitors (FLT3i), midostaurin and gilteritinib. QPOP drug combination scores and ranking were derived from analysis of cell viability response in cancer cells following treatment with a series of test combinations derived from an orthogonal array composite design. Molecular analysis was conducted to determine the accuracy of QPOP predicted response and FLT3 mutant status. Subsequently, we performed a prospective cohort pilot study of QPOP analysis in AML. AML patients from the National University Hospital,Singapore, with disease amenable to blood or marrow aspiration, were recruited between 26th November 2019 and 15th July 2022 with a median follow up of 4 months. CD33 or CD34 selection was performed to enrich for malignant blasts cells, depending on the sample. Patients were treated with current standard of care treatment options as guided by clinician opinion. The primary outcomes were to determine the ability of QPOP to predict sensitivity to FLT3i-based therapies.
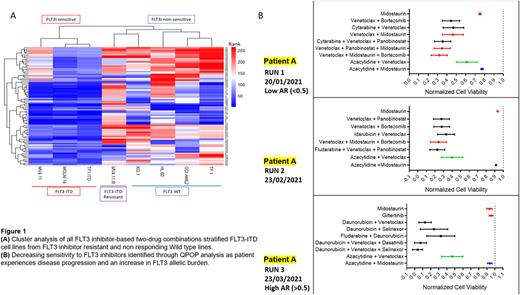
Results QPOP analysis on 8 AML cell lines was accurately able to stratify FLT3i responders and non-responders including differential responses between an FLT3-ITD isogenic parental and FLT3i resistant line. We recruited 25 patients (2 patients having repeat QPOP evaluations post treatment) with a median age of 59 years and 2 prior lines of treatment. QPOP responses were evaluable for 18 independent treatments in 15 patients (n=3 FLT3 ITD or TKD (20%), n=12 FLT3 WT (80%)). The median turn-around time for QPOP analysis was 8 (±2.1) days. Patients with either FLT3i among the top 5 ranking 2 or 3 drug combinations were suggested to be sensitive to the use of FLT3i. QPOP analysis of FLT3 mutant patients had predicted 2 patients with the FLT3-ITD mutation to be responsive to a 2-drug combination of midostaurin-venetoclax. The third FLT3-mutant patient with a TKD point mutation was predicted to be respond to a combination of gilteritinib-dasatinib. While FLT3 WT patients did not predict sensitivity to either FLT3 inhibitor as single agents or 2-drug combinations, 4/12 (33.3%) of the WT patients also showed sensitivity to the use of gilteritinib in3-drug combination rankings. This indicates that gilteritinib may be useful for FLT3 WT patients when used in the appropriate combination therapy. Furthermore, QPOP was also able to accurately identify increased sensitivity to FLT3i-based combinations in accordance with a gain of FLT3 ITD mutation. Serial QPOP analysis in subsequent samples following midostaurin-based therapy indicated a decrease in FLT3i sensitivity that was concordant with increasing FLT3 allelic burden and development of resistance. The development of resistance to FLT3i-based combinations was correlated to an increase in phosphorylated AKT expression in both AML cell lines and the patient who developed FLT3 resistance.
Conclusion QPOP analysis predicts sensitivity to drug-combinations for AML in a clinically applicable timeframe. Here we show that QPOP can be used as a tool to identify sensitivity to FLT3i-based combination therapy without prior knowledge of FLT3 mutation status. AML cell lines and patient samples can be stratified according to FLT3 inhibitor response sensitivity which parallels their mutational background and identify potential drug resistance. Further clinical study of the safety and efficacy of QPOP-derived FLT3i-based combinations as well as inquiry into the potential mechanisms of acquired resistance to these combinations will be necessary.
Disclosures
Ooi:Jenssen: Honoraria; Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Novartis: Honoraria; astellas: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria. Chow:KYAN therapeutics: Current Employment, Other: Shareholder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal